Abstract
Background: Traditional prothrombin time (PT) based international normalized ratio (INR) is often highly variable. Often this is caused by rapid factor VII reductions due to its short (4 hour) half-life. Thus, factor VII variation is an important confounder during vitamin K antagonist (VKA) dosing. Furthermore, prior studies have demonstrated that factor VII reductions only minimally influence the antithrombotic effect and bleeding risk of VKA, the clinical effect depending mainly on controlled reduction of factors II and X. Therefore, ignoring the influence of FVII during VKA monitoring has the potential to simplify management and outcome.
The Fiix prothrombin time (Fiix-PT) is a new modified PT that is only affected by reduced factors II and X. The Fiix-PT is not affected by variations in factor VII. Hence, the Fiix normalized ratio (Fiix-NR) fluctuates less than the standard standard PT-INR. In the double-blind randomized clinical Fiix-trial, published in 2015, monitoring of warfarin with with Fiix-PT (Fiix-NR) was compared to standard high time-in-range PT-INR monitoring. Fiix-NR monitoring led to improved time-in-range, reduced testing frequency, reduced dose-adjustment need and a 50% reduction in thromboembolism (TE). Bleeding did not increase and it occurred at a low rate in both trial arms. Subsequently starting on July 1st 2015 we replaced the PT-INR with Fiix-NR in our anticoagulation monitoring practice.
Aim: We compared anticoagulation outcome and dosing frequency during 12 months prior to switching from PT-INR based management (Pre-Fiix monitoring period) and during 12 months following switch to Fiix-NR monitoring (Fiix monitoring period).
Methods: This is a retrospective before and after study involving unselected patients managed at a single anticoagulation management service at the Landspitali University Hospital in Reykjavik, Iceland. All patients on warfarin during two consecutive twelve month periods were analysed irrespective of indication or anticoagulation target range. Pre-Fiix patients analysis was based on events occuring during months -12 to -1 before replacing the PT with Fiix PT and the Fiix patients´outcome was analysed during months +4 to +15. Three transitional months were excluded. All patients were dosed by specialized staff using the DAWN anticoagulation software®. Hospital chart diagnostic codes were reviewed for occurrence of TE or major bleeding (MB) during the two separate monitoring periods.Surrogate assessments included the number of tests, testing intervals and dose adjustment frequencies and will be analyzed later.
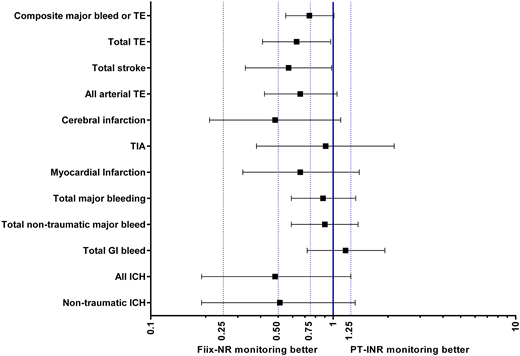
Results: During the Pre-Fiix period 2,345 patients were managed with PT-INR for 1,984 patient years (0.85 years/patient) and during the Fiix period 1,909 patients were managed with Fiix-NR for 1,643 patient years (0.86 y/pt). Indications for warfarin were identical during both periods (atrial fibrillation 57%, venous thromboembolism 26%, prosthetic heart valves 5% and other 12%). The INR target range during both periods was 2.0-3.0 in 92% and 2.5-3.5 in 6%. Clinical outcome with respect to TE and MB is shown in the figure. Overall the point estimates were suggestive of a 34-52% reduction of TE with Fiix-NR monitoring whereas major bleeding was similar. The reduction in untoward events in the Fiix group was statistically significant for composite new TE (OR 0.63;0.41-0.97) and total stroke (ischemic and hemorrhagic, OR 0.57;CI 0.33-0.98), mainly driven by reduced cerebral infarction. Intracranial bleeding occurred in 0.76% in the Pre-Fiix group and in 0.37% in the Fiix montoring group (n.s., OR 0.51;0.19-1.32). The median time-within-Target-range (TTR, Rosendaal method) was similar in both groups (75 vs 76%, respectively), despite dose adjustment frequency being reduced by 18% in the Fiix monitoring group (OR 0.82;CI 0.79-0.85, p <0.0001).
Conclusions: These results are in agreement with the results of the Fiix-trial and show that ignoring factor VII during VKA monitoring is safe and leads to reduction in thromboembolism without increasing bleeding. Although TTR was identical in both groups, the dose adjustment need was reduced possibly indicating that less anticoagulation variability in the Fiix-NR group explains reduced thromboembolism.
Gudmundsdottir:Hart Biologicals Ltd: Consultancy, Patents & Royalties: Hart Biologicals Ltd is commercializing the Fiix-PT which will be ready for marketing in Europe in the beginning of year 2019 and possibly later that year in the USA. Patent fees will be paid to Fiix Diagnostics Ltd once the test is commercialized.; Fiix Diagnostics Ltd: Equity Ownership, Patents & Royalties: The Fiix prothrombin time (Fiix-PT) is a patented invention of P. T. Onundarson and B.R. Gudmundsdottir that is owned by Fiix Diagnostics Ltd, a start-up company founded by the inventors. . Onundarson:Hart Biologicals Ltd: Consultancy, Other: Hart Biologicals Ltd is commercializing the Fiix-PT which will be ready for marketing in Europe in the beginning of year 2019 and possibly later that year in the USA. Patent fees will be paid to Fiix Diagnostics Ltd once the test is commercialized.; Fiix Diagnostics Ltd: Equity Ownership, Patents & Royalties: The Fiix prothrombin time (Fiix-PT) is a patented invention of P. T. Onundarson and B.R. Gudmundsdottir that is owned by Fiix Diagnostics Ltd, a start-up company founded by the inventors. .
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal